In the rapidly evolving landscape of cancer immunotherapy, a new contender is steadily gaining prominence for its potential to overcome longstanding challenges in treating solid tumors. CAR-NK cell therapy, an innovative approach that merges the targeted precision of chimeric antigen receptors with the innate biological advantages of natural killer cells, is emerging as a promising strategy where other immunotherapies have struggled. Unlike its more famous counterpart CAR-T, which has revolutionized blood cancer treatment but faces significant hurdles in solid malignancies, CAR-NK therapy brings a unique set of properties that may finally crack the code of complex tumor environments.
The fundamental challenge with solid tumors has always been their hostile microenvironment—a complex ecosystem of physical barriers, immunosuppressive signals, and metabolic challenges that effectively disarm incoming immune cells. CAR-T cells, while powerful, often become exhausted or inhibited upon encountering these conditions. They also carry risks of severe side effects like cytokine release syndrome and require personalized manufacturing from the patient’s own T cells, making treatment costly and logistically complex. Natural killer cells, in contrast, offer a compelling alternative. They are innate immune cells designed to recognize and eliminate abnormal cells without prior sensitization, and they do so without triggering the same severe inflammatory cascades.
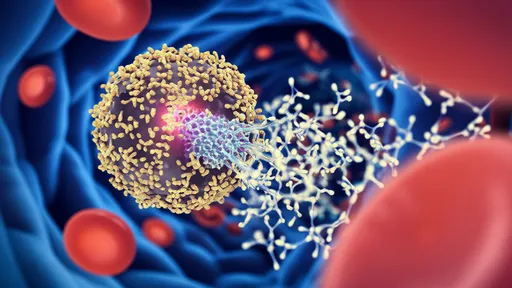
What makes CAR-NK therapy particularly intriguing is the synergy between the natural tumor-fighting instincts of NK cells and the engineered specificity provided by the chimeric antigen receptor. By equipping NK cells with a CAR that targets a specific tumor antigen—such as HER2 in breast cancer or GD2 in neuroblastoma—researchers can direct these cells precisely to cancer cells while leveraging their innate ability to kill through multiple mechanisms. This includes the release of cytotoxic granules containing perforin and granzymes, death receptor-mediated apoptosis through ligands like FASL and TRAIL, and the secretion of inflammatory cytokines that recruit other immune cells to the tumor site.
Several recent preclinical and early-phase clinical studies have demonstrated encouraging activity of CAR-NK cells against various solid tumors. In ovarian cancer models, for instance, CAR-NK cells targeting the mesothelin antigen have shown potent tumor cell killing and an ability to suppress tumor growth significantly. In glioblastoma, one of the most aggressive and treatment-resistant solid tumors, CAR-NK cells designed to target EGFRvIII or HER2 have exhibited robust antitumor responses and an impressive capacity to infiltrate the brain tumor microenvironment. Perhaps most notably, these responses often occur without the severe cytokine-driven toxicities commonly associated with CAR-T therapy.
The off-the-shelf potential of CAR-NK therapy represents another significant advantage. While CAR-T cells are typically autologous (derived from the patient themselves), NK cells can be sourced from multiple allogeneic origins—peripheral blood, cord blood, or even induced pluripotent stem cells—without triggering dangerous graft-versus-host disease. This means CAR-NK products could be manufactured in large, standardized batches, stored, and made readily available for patients, dramatically reducing costs and wait times compared to personalized therapies. Companies are already developing master cell lines that could provide a renewable source of therapeutic NK cells, potentially making this treatment accessible to much larger patient populations.
Despite these promising advantages, CAR-NK therapy for solid tumors still faces considerable challenges. The immunosuppressive tumor microenvironment remains a formidable barrier, with factors like TGF-β, adenosine, and prostaglandins that can inhibit NK cell function. Physical barriers like dense extracellular matrix and abnormal tumor vasculature can prevent CAR-NK cells from reaching their targets in sufficient numbers. Tumor antigen heterogeneity—where not all cancer cells express the target antigen—poses another significant obstacle, as antigen-negative cells can escape recognition and lead to treatment resistance.
Researchers are addressing these challenges through sophisticated engineering strategies. Next-generation CAR-NK designs incorporate features to enhance persistence, overcome immunosuppression, and target multiple antigens simultaneously. Some constructs include cytokine genes like IL-15 to support NK cell survival and proliferation within the tumor. Others are engineering resistance to inhibitory factors like TGF-β or adding chemokine receptors to improve tumor homing. Dual-targeting CARs and tandem CAR designs that recognize two different tumor antigens are being developed to prevent antigen escape, while safety switches provide mechanisms to control the therapy if needed.
The clinical translation of CAR-NK therapy is accelerating, with several trials now underway for solid tumors. Phase I studies are investigating CAR-NK cells against pancreatic cancer, breast cancer, brain tumors, and other malignancies, with initial results expected in the coming years. While still early, the safety profile appears favorable, with most reported adverse events being manageable and less severe than those observed with CAR-T therapy. Efficacy signals, while preliminary, have been encouraging enough to warrant expanded studies and combination approaches with other treatments.
Looking forward, the future of CAR-NK therapy likely lies in rational combination strategies. Pairing CAR-NK cells with checkpoint inhibitors, cancer vaccines, or targeted therapies could create synergistic effects that enhance antitumor immunity. Radiation or chemotherapy might be used to condition the tumor microenvironment, making it more permissive to NK cell attack. The flexibility of NK cell biology and genetic engineering suggests that we've only begun to scratch the surface of what's possible with this platform.
As research progresses, CAR-NK therapy holds the potential to transform the treatment paradigm for solid tumors. While challenges remain, the unique biological properties of NK cells—combined with advances in genetic engineering and manufacturing—are creating unprecedented opportunities to develop effective, safe, and accessible immunotherapies for cancers that have long eluded successful treatment. The coming years will be critical in determining whether CAR-NK cells can fulfill their promise and become a mainstream option for patients with solid malignancies.
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025