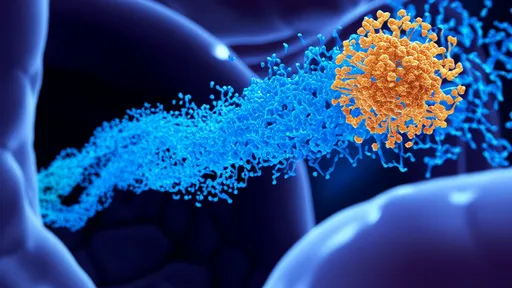
In the rapidly evolving landscape of cancer immunotherapy, a groundbreaking development has emerged from the laboratories of immunologists and bioengineers worldwide. The focus has shifted toward overcoming one of the most persistent challenges in adoptive cell therapies: host immune rejection of therapeutic cells. Universal CAR-T cells, engineered to be invisible to the patient’s immune system, are poised to revolutionize the field, offering hope for more accessible, scalable, and effective treatments.
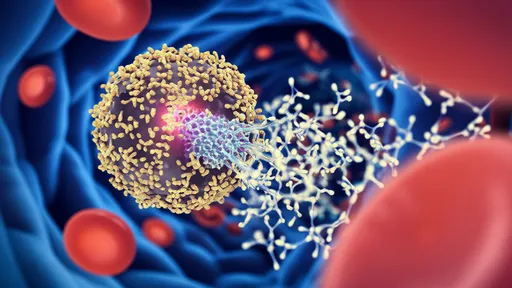
The concept of chimeric antigen receptor T-cell therapy, or CAR-T, has already transformed the treatment of certain blood cancers. By reprogramming a patient’s own T cells to recognize and attack cancer cells, CAR-T therapy has achieved remarkable success in cases where conventional treatments have failed. However, this personalized approach is fraught with limitations. Each treatment must be manufactured individually from the patient’s own cells, a process that is not only time-consuming and costly but also unfeasible for patients with compromised immune systems or insufficient T-cell counts.
To address these constraints, researchers turned to the idea of allogeneic, or “off-the-shelf,” CAR-T cells derived from healthy donors. These universal CAR-T cells could be mass-produced, stored, and made readily available for any patient, drastically reducing preparation time and cost. But this approach introduced a new hurdle: graft-versus-host disease (GvHD), where the donor T cells attack the patient’s healthy tissues, and host-versus-graft rejection, where the patient’s immune system recognizes and destroys the foreign CAR-T cells.
Immunoengineering has risen to this challenge with innovative strategies to cloak these universal cells from immune detection. One of the most promising techniques involves the use of gene editing tools like CRISPR-Cas9 to knock out genes responsible for the expression of T-cell receptor (TCR) complexes. Without TCRs, the donor T cells are unable to recognize the patient’s tissues, thereby preventing GvHD. Simultaneously, to evade host immune rejection, scientists are deleting genes that code for major histocompatibility complex (MHC) molecules, which act as flags that identify cells as foreign.
However, removing MHC molecules creates a new problem: without these signals, the engineered T cells might become vulnerable to attack by the patient’s natural killer (NK) cells, which monitor for missing or altered MHC expression. To counter this, researchers are incorporating synthetic molecules or engineering the cells to express non-native, inhibitory ligands that effectively signal to NK cells, “do not attack.” This delicate balance of edits aims to create stealth T cells that can operate undetected in any recipient.
Recent preclinical studies have demonstrated the viability of these approaches. In animal models, universal CAR-T cells with TCR and MHC knockouts, supplemented with NK evasion tactics, have shown sustained persistence and potent antitumor activity without eliciting rejection or GvHD. These findings have paved the way for early-phase clinical trials, where initial results are being closely watched for safety and efficacy signals.
Beyond genetic modifications, some groups are exploring alternative methods to achieve universal compatibility. This includes encapsulating CAR-T cells in biomaterials that shield them from immune recognition or using small molecule drugs to temporarily suppress the host immune response during cell administration. While these strategies are still in nascent stages, they represent the creative breadth of immunoengineering aimed at making CAR-T therapy more universal.
The implications of successful universal CAR-T cells extend beyond logistics and cost. They could enable treatment for a broader patient population, including those in resource-limited settings where personalized cell therapy is impractical. Moreover, universal platforms facilitate rapid iteration and improvement of CAR designs, as a single engineered cell line can be tested and optimized across multiple contexts, accelerating the development of next-generation therapies.
Despite the excitement, significant challenges remain. Ensuring long-term safety, particularly the risk of off-target effects from gene editing, is paramount. Regulatory pathways for these complex, multiply engineered products are still evolving, requiring close collaboration between scientists, clinicians, and policymakers. Furthermore, the durability of response and potential for antigen escape—where tumors stop expressing the target antigen—are ongoing concerns that must be addressed through combination strategies or more sophisticated CAR designs.
As the field advances, the vision of a truly universal, off-the-shelf cell therapy is inching closer to reality. Immunoengineering has provided the tools to reimagine CAR-T not as a bespoke treatment, but as a standardized, scalable pharmaceutical product. This paradigm shift holds the promise of democratizing access to cutting-edge cancer immunotherapy, bringing us one step closer to a future where life-saving cell therapies are available to all who need them.
The journey of universal CAR-T cells from bench to bedside exemplifies the power of interdisciplinary innovation. By marrying immunology with genetic engineering, materials science, and synthetic biology, researchers are overcoming biological barriers that once seemed insurmountable. As clinical data accumulates and technologies mature, these engineered cells are set to redefine what is possible in the fight against cancer and beyond.
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025