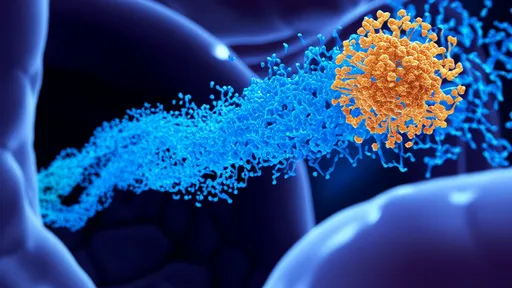
In a landmark development that could reshape the landscape of oncological therapeutics, researchers have unveiled a groundbreaking approach utilizing DNA origami-based drug delivery systems to target tumors with unprecedented precision. This innovative strategy harnesses the programmable nature of DNA to construct nanoscale carriers capable of navigating the complex biological environment to deliver chemotherapeutic agents directly to malignant cells, thereby minimizing systemic toxicity and enhancing treatment efficacy. The implications of this technology extend far beyond conventional chemotherapy, offering a glimpse into a future where cancer treatment is both highly effective and remarkably gentle on the patient.
The foundation of this breakthrough lies in the field of DNA nanotechnology, particularly DNA origami, which involves the folding of long single-stranded DNA molecules into precise two- and three-dimensional shapes through hybridization with shorter staple strands. Scientists have engineered these nanostructures to function as sophisticated cargo ships, designed to transport anticancer drugs such as doxorubicin or siRNA molecules. What sets these carriers apart is their ability to be functionalized with various targeting ligands, including aptamers or antibodies, that recognize and bind to specific receptors overexpressed on the surface of tumor cells. This ensures that the therapeutic payload is released predominantly at the disease site, drastically reducing off-target effects.
Recent in vivo studies have demonstrated the remarkable potential of these systems. In murine models of aggressive breast cancer and glioblastoma, DNA origami carriers loaded with chemotherapeutic agents showed a significant reduction in tumor growth compared to free drug administration. Histological analyses revealed substantially less damage to healthy tissues, particularly the heart and liver, which are commonly affected by traditional chemotherapy. The targeted nature of the delivery was confirmed using fluorescence imaging, which showed a strong accumulation of the nanostructures within tumors, with minimal distribution in other organs. This targeted accumulation is facilitated by the enhanced permeability and retention effect, a phenomenon where certain sizes of molecules tend to accumulate in tumor tissue more than in normal tissues due to leaky vasculature and poor lymphatic drainage.
Overcoming the biological barriers has been a central challenge in nanomedicine. The human body is equipped with a robust defense system designed to identify and eliminate foreign particles. The mononuclear phagocyte system, primarily in the liver and spleen, often clears nanoparticles from the bloodstream before they can reach their intended target. However, the DNA origami structures in these latest iterations have been coated with stealth polymers like polyethylene glycol, which effectively disguises them from immune surveillance, prolonging their circulation time and increasing their chances of reaching the tumor vasculature. Furthermore, the precise control over the size and shape afforded by DNA origami allows for optimization of these parameters to maximize tumor penetration and cellular uptake.
The versatility of the DNA platform is another of its most compelling attributes. Unlike many synthetic nanoparticles, which can be difficult to modify consistently, DNA nanostructures can be designed with atomic-level precision using computer-aided software. Researchers can easily incorporate different functional elements—such as multiple targeting moieties, imaging contrast agents for tracking, and even stimuli-responsive mechanisms for controlled drug release—all within a single, unified structure. For instance, some designs include sequences that are sensitive to the acidic pH of the tumor microenvironment or to specific enzymes present there, ensuring that the drug is only released upon arrival at the cancer site.
Despite the exhilarating progress, the path to clinical translation is paved with significant hurdles that must be diligently addressed. The scale-up production of these complex DNA nanostructures under Good Manufacturing Practice conditions presents a substantial economic and technical challenge. Questions regarding long-term stability, potential immunogenicity, and the fate of the DNA framework within the body after drug release are active areas of investigation. Early toxicology studies are encouraging, showing that these structures made from natural DNA nucleotides are generally well-tolerated, but comprehensive studies are essential before human trials can commence. The scientific community is actively exploring these questions, with several biotech startups and pharmaceutical giants investing heavily in the technology.
The convergence of biology and nanotechnology in this manner represents a paradigm shift in how we approach disease treatment. It moves us away from the blunt instrument of systemic drug administration towards a era of intelligent, programmable medicine. The success of DNA origami drug carriers against tumors is not just a victory for cancer therapy; it serves as a powerful proof-of-concept for the entire field of targeted nanomedicine. It demonstrates that we can engineer biological molecules to perform complex, pre-designed tasks within the human body, opening the door to treating a myriad of other diseases with similar precision.
As research accelerates, the first-in-human trials for such DNA origami systems are likely on the horizon within the next five to seven years. The data continues to mount, overwhelmingly supporting the efficacy and safety of this approach in preclinical models. The journey from a laboratory concept to a standard treatment option is long and arduous, but the potential reward—a highly effective, targeted therapy that drastically reduces the suffering associated with cancer treatment—is a powerful motivator for scientists and clinicians worldwide. This isn't merely an incremental improvement; it is a fundamental reimagining of drug delivery that promises to redefine our battle against cancer.
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025