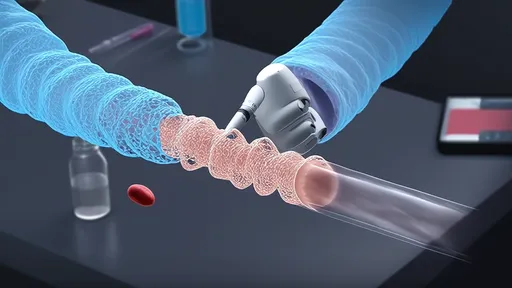
In the rapidly evolving field of nanotechnology, scientists have achieved a groundbreaking milestone with the development of DNA nanocalipers—a revolutionary tool capable of manipulating proteins within living cells. This innovation opens up unprecedented possibilities for precise cellular engineering, offering researchers the ability to probe, measure, and control molecular interactions in real time. Unlike traditional methods, which often lack precision or disrupt cellular functions, DNA nanocalipers operate with remarkable accuracy, leveraging the programmable nature of DNA to interact with specific proteins without harming the cell.
The concept of using DNA as a building material for nanoscale devices isn’t entirely new, but its application as a functional tool inside living cells marks a significant leap forward. DNA nanocalipers are designed to mimic the behavior of mechanical calipers at the molecular level. These structures can open and close in response to specific triggers, such as changes in pH or the presence of certain molecules, allowing them to grasp or release target proteins with exceptional specificity. This level of control is particularly valuable in studying protein-protein interactions, which are fundamental to understanding cellular processes like signaling, metabolism, and disease progression.
What sets DNA nanocalipers apart is their ability to function within the complex and dynamic environment of a living cell. Traditional protein manipulation techniques often require isolating proteins from their natural context, which can alter their behavior and lead to misleading results. By contrast, DNA nanocalipers work in situ, enabling researchers to observe and manipulate proteins as they perform their native functions. This capability is already yielding insights into previously inaccessible aspects of cell biology, such as the real-time dynamics of enzyme activity or the mechanical forces exerted by motor proteins.
The design of DNA nanocalipers hinges on the principles of DNA origami, a technique that folds long strands of DNA into precise shapes. Scientists engineer these structures to include binding sites that recognize and attach to specific proteins. Once inside the cell, the nanocalipers can be activated to clamp down on their targets, either to hold them in place or to bring them into proximity with other molecules. This precision is akin to using a pair of tweezers at the nanoscale, but with the added advantage of being programmable and reversible.
One of the most promising applications of this technology lies in the field of targeted therapeutics. By programming DNA nanocalipers to interact with disease-related proteins, researchers could potentially develop new treatments that intervene at the molecular level. For example, in cancer cells, nanocalipers could be designed to block the activity of proteins that drive uncontrolled growth or to deliver drugs directly to malignant cells while sparing healthy tissue. Such approaches could minimize side effects and improve the efficacy of treatments.
Beyond medicine, DNA nanocalipers could also transform synthetic biology. Engineers could use these tools to assemble custom protein complexes or to rewire cellular pathways, creating cells with novel functions. Imagine designing immune cells that are hyper-responsive to pathogens or reprogramming stem cells to differentiate into specific tissue types with higher efficiency. The possibilities are vast, and the technology is still in its early stages, with researchers only beginning to explore its full potential.
However, challenges remain in scaling up the use of DNA nanocalipers for broader applications. Delivering these nanostructures into cells consistently and ensuring their stability in diverse cellular environments are active areas of research. Additionally, while DNA is biocompatible, its introduction into cells could still trigger immune responses or unintended interactions with other biomolecules. Addressing these hurdles will be critical for translating laboratory successes into practical tools for medicine and industry.
Despite these obstacles, the development of DNA nanocalipers represents a paradigm shift in how scientists interact with the molecular machinery of life. By bridging the gap between nanotechnology and cell biology, this innovation promises to unlock new frontiers in research and therapy. As the technology matures, it may well become a staple tool in the life sciences, much like CRISPR has for gene editing. For now, the scientific community watches with anticipation as each new study brings us closer to harnessing the full power of this remarkable invention.
By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025